An Introduction to the Enzyme-Linked Immunosorbent Assay – ELISA Test

Complete the form below to unlock access to ALL audio articles.
There are many instances within the life sciences where detection and quantification of antigens or antibodies within a sample in a timely and cost-effective manner is important. From identifying immune responses in vaccinated or infected individuals, detecting expression of a protein you wish to express on the surface of a cell, to performing quality control testing. Having a tool capable of making such assessments is key.
The enzyme-linked immunosorbent assay (ELISA) is one such test that has proven invaluable as both a research and diagnostic tool. In this article, we will consider what an ELISA is, how it works, variations of the technique and what it can tell you.
Types of ELISA and ELISA test diagram
- Direct ELISA test
- Indirect ELISA test
- Sandwich ELISA
- Competitive ELISA
ELISA steps
ELISA test results, what does a positive ELISA test tell you?
Applications of ELISAs and the LAM ELISA test
What is an ELISA test?
An ELISA is an immunological assay commonly used to measure antibodies or antigens, including proteins or glycoproteins, in biological samples. Like other immunoassays, they rely on binding of antibodies to their targets to facilitate detection.
Typically, ELISA assays are performed in 96-well plates, a format that makes them amenable to screening many samples at once. Serum, plasma, cell culture supernatants, cell lysates, saliva, tissue lysates and urine are all common sample types used for these assays, but most liquid sample types could be used in theory. It is, however, important to consider that some sample types may include inhibitory factors, such as buffer components that share similar antigenic epitopes1 or factors like proteases2 that may damage the target or detection components, that may interfere with the assay’s performance.
There are several different assay formats, but all rely on binding of either the target itself or an antibody/antigen able to capture the target to the surface of the plate. A detection step involving a conjugated antigen, or more often antibody, is then employed to enable successful binding to be detected and quantified, most often by colorimetric detection.
Types of ELISA and ELISA test diagram
The ELISA was originally conceptualized, independently, in 1971 by Eva Engvall and Peter Perlman3 at Stockholm University in Sweden, and Anton Schuurs and Bauke van Weemen4 in the Netherlands. They sought an immunoassay method able to detect the presence of antigens or antibodies to replace the radioimmunoassay, which employed potentially hazardous radioactively labeled antigens or antibodies, and thus devised an enzyme-based alternative.
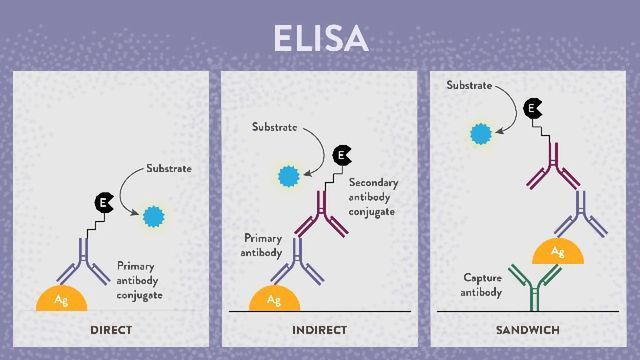
There are now four main types of ELISA, direct, indirect, sandwich and competitive. The images below (Figure 1) illustrate detection of antigens; however, the same principle applies for antibody detection essentially with the roles of the antigen and primary antibody reversed.
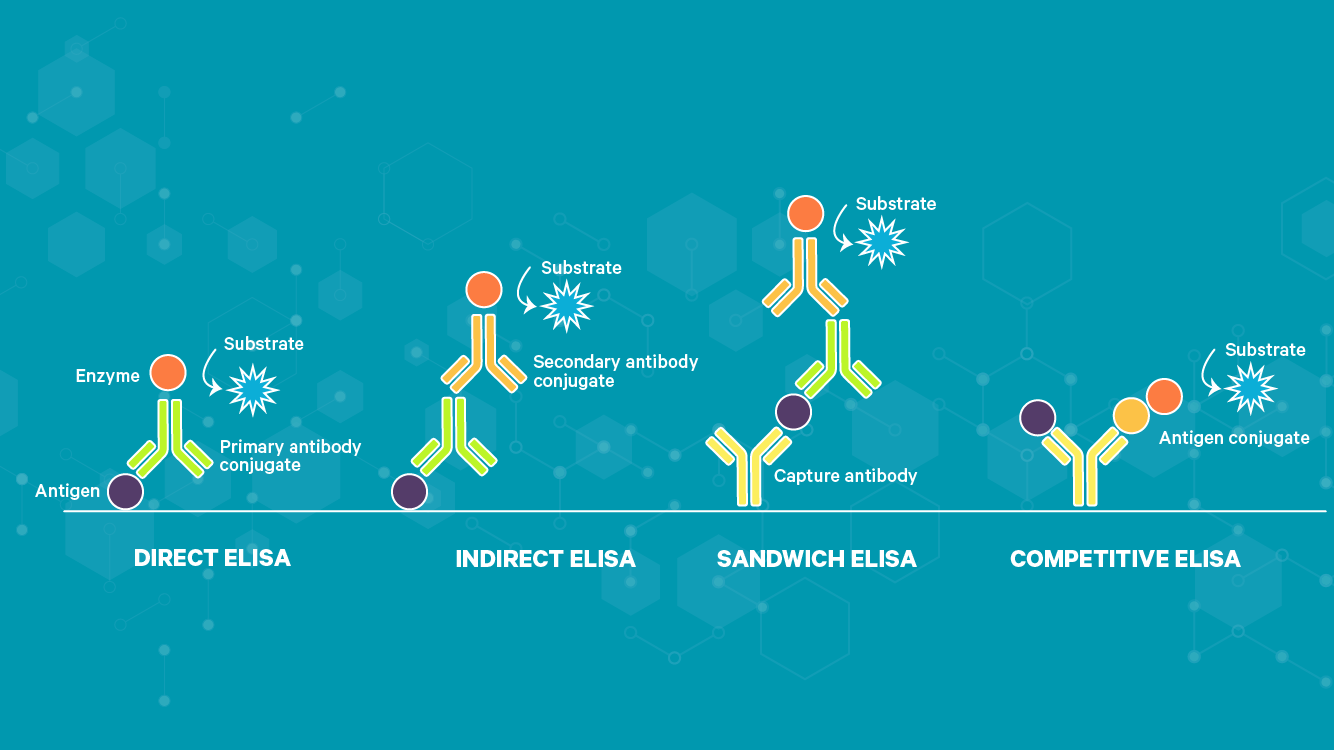
Figure 1: Types of ELISA.
Direct ELISA test
With a direct ELISA, the antigens or antibodies in a sample are adsorbed directly to the test plate in a non-specific manner. A conjugated detection antibody or antigen specific for the target is then applied to the wells. Following this, a detection substrate is used to produce a measurable color change that can be quantified in a plate reader.
As this assay has few steps, it is quicker and offers less opportunity for the introduction of errors than the other ELISA methods. However, as the adsorption step is non-specific, background noise may be high. The absence of a secondary antibody step means there is no signal amplification, reducing assay sensitivity. It also requires conjugated detection antibodies/antigens to be created for each target required.
Indirect ELISA test
Originally developed in 19785 for the detection of human serum albumin, the indirect ELISA, or iELISA, works in a very similar way to the direct ELISA except for the addition of a secondary antibody step. This enables amplification of the test signal, overcoming the limitation of the direct ELISA. It also negates the need for target-specific conjugated detection antibodies/antigens as the conjugated secondary antibody need only be species specific for the primary antibody. Where total sample antigens are bound to the plates, like the direct ELISA, background noise remains an issue. However, if the assay is used for the detection of sample antibodies, purified target antigen is coated onto the plates, with the primary antibody coming from the sample. This greatly reduces background noise and consequently these assays are most popular for determining antibody titers in samples.
Disadvantages of the indirect ELISA include a longer protocol with more opportunities for errors and potential of cross-reactivity with the secondary antibody.
Sandwich ELISA
Developed in 19776, as its name suggests, the sandwich ELISA sandwiches the antigen between antibodies. The technique can employ the direct or indirect ELISA format (the sandwich ELISA depicted above is based on the indirect ELISA) except that rather than non-specific binding of antigens to the assay plate, the capture antibody makes this a specific process. This combination further improves assay sensitivity and specificity.
They do, however, require the determination of compatible capture and detection antibody pairs to function effectively with which cross-reactivity can be problematic. This assay typically has the most steps too, offering greater opportunity for error. Due to the selective nature of the antigen binding step, sandwich ELISAs are particularly useful where the antigen is in a complex mix as antigen purification is not required.
Competitive ELISA
A competitive ELISA, also known as an inhibition ELISA or blocking ELISA, is possibly the most complex of the ELISA techniques. Originally developed in 19767 for the detection of human choriogonadotropin, the assay works by detecting interference to an expected output signal level, producing an inverse relationship. The more of the target there is in the sample applied, the lower the assay output signal will be. Multiple formats are possible for which the other ELISA types can be adapted into a competitive format. However, there are two general principles; the sample antigen or antibody competes with a reference for binding to a limited amount of labeled antibody or antigen, respectively. Alternatively, sample antigen or antibody may compete with a labeled reference for binding to a limited amount of antibody or antigen, respectively.
The competitive ELISA will have some of the same advantages and limitations as the format from which it has been adapted. However, it can be helpful when the antigen is small, limiting the ability of two antibodies to bind concurrently, as required for the sandwich ELISA, or when only one antibody is available.
ELISA steps
While there are variations in the protocols for the different types of ELISA, there are a number of stages to the assay common to most that should be considered.
Plate coating
The first step in most ELISAs is the binding of the first component of the assay to the test plate. This is often done through passive adsorption, which is a non-specific process, so the result will depend on what is applied to the plate. If antigen-containing sample is applied, then the result is a plate for which selective steps are still required as a whole variety of antigens will bind, not just those of interest. If, however, purified antigen — as in the case of an indirect ELISA for antibody detection — or capture antibody — as in the case of the sandwich ELISA — is applied, then the result is a plate that already has selective properties.
Multiple plate types, most of which are polystyrene or polystyrene derivatives, with differing binding properties are available depending on the target and nature of the assay. Some targets, including heavily glycosylated proteins, carbohydrates, DNA, lipids, short peptides and proteins in the presence of detergents, do not adsorb well. In these cases, it is advisable to use plates that have been treated to permit covalent linkage to the surface instead.
Incubations
Following the addition of reagents to the ELISA plates, they must be incubated to allow time for binding or reactions to take place. The temperature and duration of each incubation will depend upon the assay step and the action being performed. Incubation for 1 hour at 37 °C is commonly used, for example following sample application. However, steps like blocking may be performed overnight in a fridge and incubation during detection is often performed at room temperature for much shorter times.
Washing
Plate washing is a vital step between the application of each component of the assay right up until detection. After solutions are emptied from the wells, the wells are washed with a buffer — often phosphate-buffered saline-Tween 20 (PBST) — to remove any residual unbound antigens, antibodies or reagents that remain. This may be done by hand with a multichannel pipette or using an automated plate washer. Failure to wash sufficiently may result in high background signal, whereas too much washing can result in low sample signals. Inconsistent washing is likely to introduce inconsistencies across the plate, resulting in unreliable results.
Blocking
Following the coating of ELISA plates with proteins, blocking is often necessary to prevent any non-specific binding of detection antibodies in the following protocol steps (Figure 2). Mixed proteins unrelated to the assay are added to and incubated in the plate, occupying any available non-specific binding sites. Common protein blocking buffer choices include skimmed dried milk, bovine serum albumin (BSA) and casein. Alternatively, nonionic detergents, such as Tween 20 or Triton X-100, may be used as blocking agents but do not provide permanent blocking like proteins. Ineffective plate blocking can lead to increased background noise and reduce assay sensitivity and specificity.
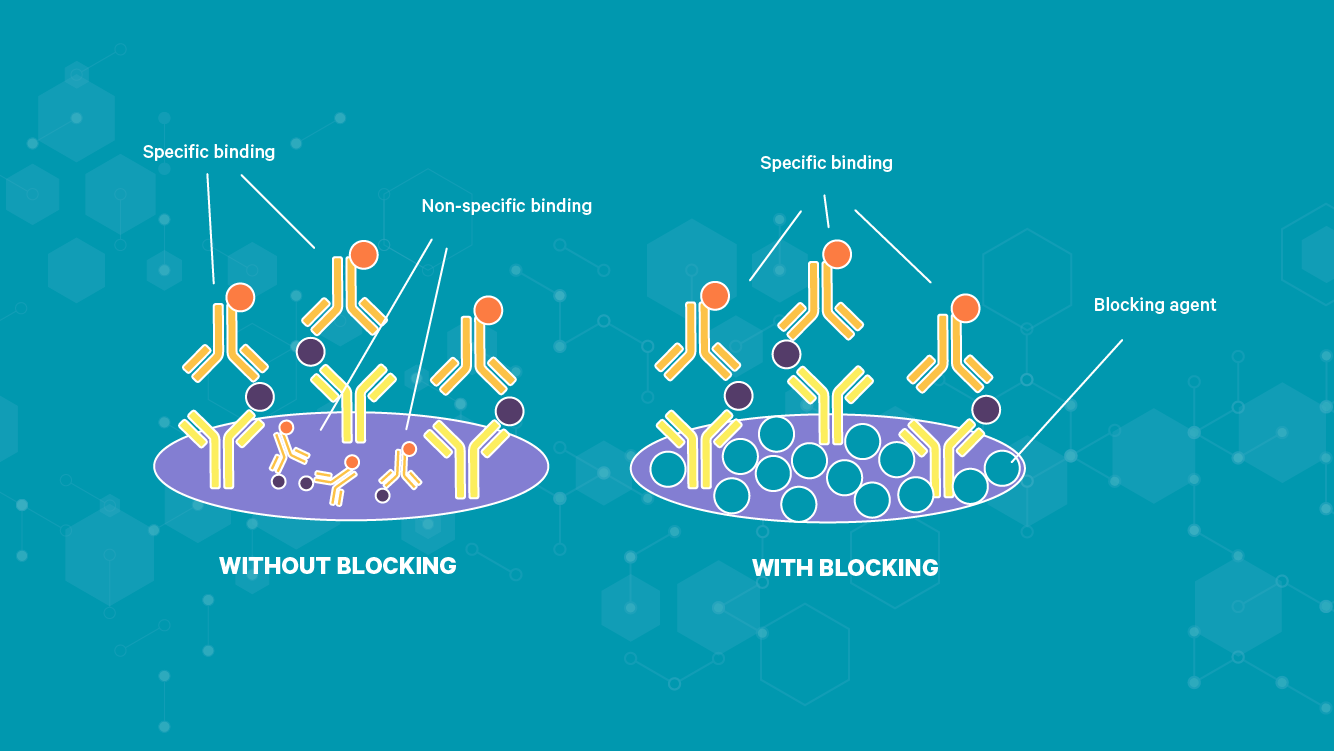
Figure 2: How the blocking process prevents non-specific binding.
Antibodies
Experimental antibodies are the cornerstone of most ELISAs and it is of paramount importance to choose the right ones, especially where multiple antibodies are used. Both monoclonal and polyclonal antibodies can be used, each with their own advantages and limitations. Monoclonal antibodies offer high specificity but are more costly. Polyclonal antibodies on the other hand can bind a target at multiple binding sites, amplifying the signal and improving sensitivity.
The use of a methods employing a secondary antibody adds extra steps, prolonging the assay time, increasing the opportunity for mistakes and requiring more optimization to find an appropriate compatible antibody pair. However, sensitivity gains from the use of a polyclonal secondary antibody may make this worthwhile or essential to the development of an effective assay.
Detection
Irrespective of the ELISA type used, all end in a detection step, most often utilizing enzyme-mediated visible color change chemistry which can then be measured using UV-Vis spectrophotometry. An enzyme-conjugated antigen or antibody is applied to test wells where it will bind if its target is present. When an appropriate substrate for the enzyme is added to the plate, it causes a color change that is proportional to the amount of target bound inside the well. Horseradish peroxidase (HRP) is a common conjugate used in partnership with the substrate 3,3',5,5'-tetramethylbenzidine (TMB), which turns blue in response to HRP and then yellow on the addition of a sulfuric acid solution that stops the reaction.
The absorbance values for each well can then be determined using a microplate reader (at 450 nm in the case of TMB following the addition of stop solution) — a type of UV-Vis spectrophotometer — and corrections and calculations, such as subtraction of average blank well values, averaging of technical replicates or ratio calculation to standards, made according to the assay design.
An example of an ELISA workflow are depicted in Figure 3.
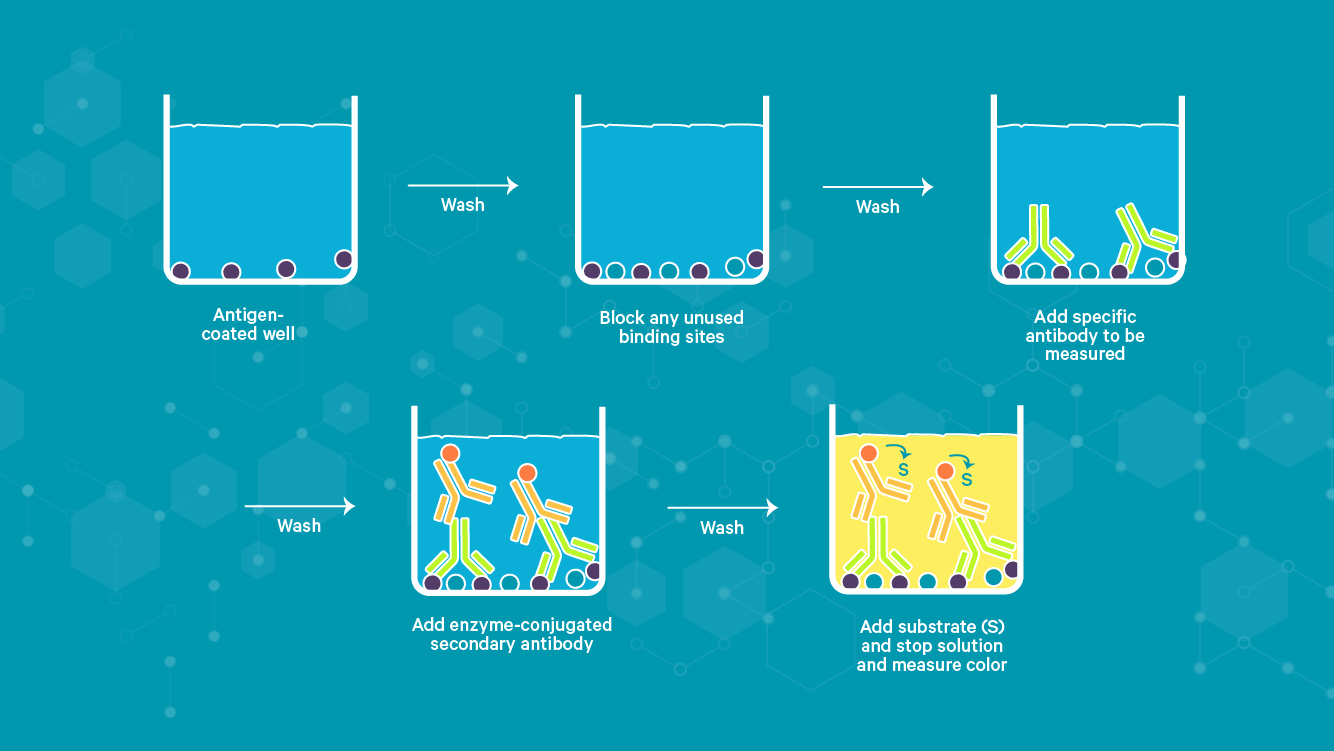
Figure 3: Example of an iELISA workflow.
ELISA test results, what does a positive ELISA test tell you?
ELISA results may be interpreted quantitatively, qualitatively or semi-quantitatively. In a quantitative assay, a serial dilution of a known standard is used to enable the generation of a standard curve, normally of optical density (OD) versus concentration. From this, the precise quantities of target in the unknown samples can be calculated (Figure 4).
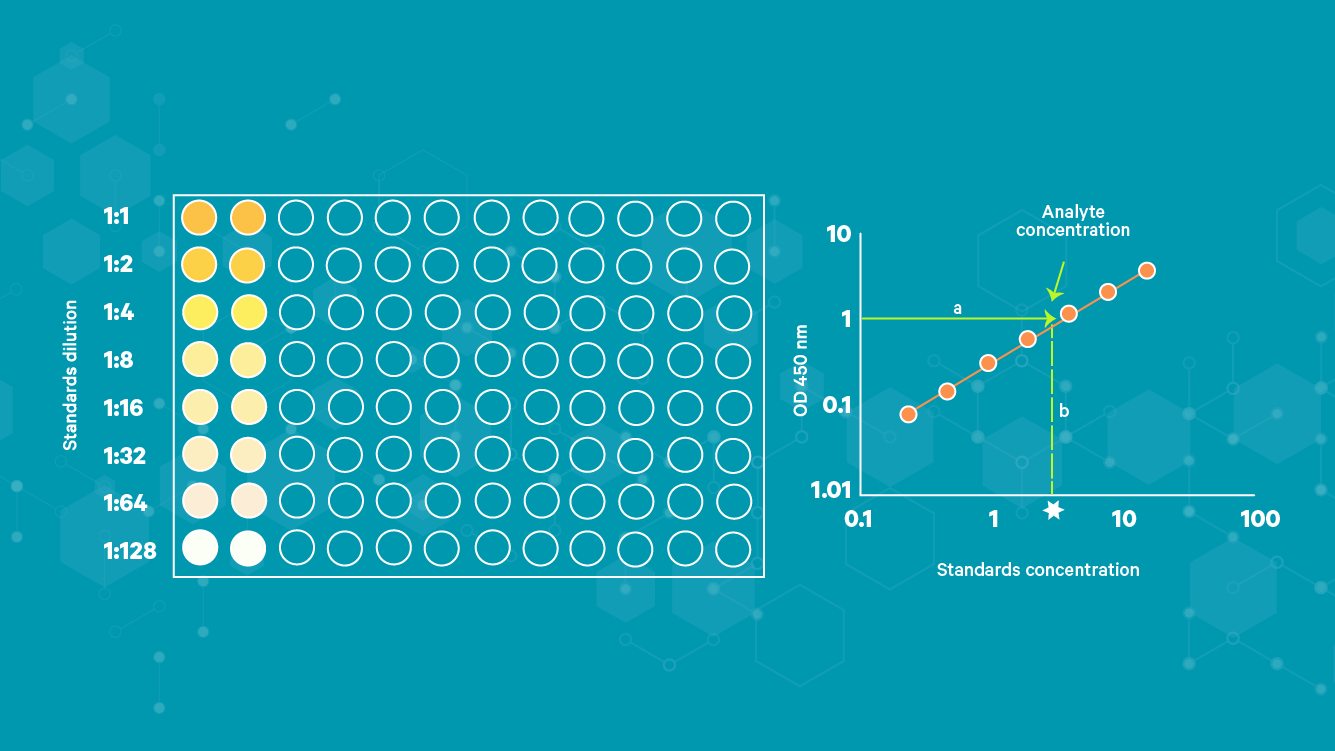
Figure 4: Example of standard curve calculation and target concentration determination of an unknown for an ELISA experiment.
In a qualitative assay, data will still normally be collected numerically from the use of a microplate reader, but results will be interpreted as positive or negative by comparison to blank and/or negative wells with no standard curve.
Semi-quantitative assays also collect numerical data without the use of a serial dilution or standard curve. However, the inclusion of both negative and positive standards, often high and low positives, facilitates the relative comparison of levels between the wells of unknown samples to those of known status. With the use of these standards to monitor assay reproducibility, a cut-off value may be set with results over the threshold determined to be positive and those below negative. Some assays may also incorporate an “amber zone”. For samples whose values fall in this region, retesting or further investigation is recommended.
While a quantitative assay may be desirable in some settings, the inclusion of a full standard curve on each plate takes up valuable wells. Therefore, a trade-off exists depending on the information the user hopes to obtain from the assay result.
Like many assays, the results obtained from an ELISA are rarely likely to be 100% accurate, with both false positive and false negative results a possibility. Consequently, most tests will be associated with measures of sensitivity and specificity; the closer they are to 100%, the better the test.
There are many factors that can impact these values such as:
- High background from non-specific binding or cross-reactivity
- Poor affinity of antibodies for their targets
- Suboptimal assay conditions
- Sample condition and complexity
Calculating sensitivity and specificity values is therefore an important step in the development of many ELISA assays, particularly in a diagnostic setting, to determine how informative and how reliable any results obtained are.
Applications of ELISAs and the LAM ELISA test
Since its conception, this flexible and inexpensive test has found many uses and continues to do so. Some of these applications are highlighted below.
Infectious disease diagnostics
The ELISA is particularly popular in diagnostic testing where is can be applied to the detection of both the infectious agents themselves and the antibody response they induce in the host, depending on the assay conformation chosen. Consequently, ELISAs can be useful in both identifying infected individuals that may pose an infection threat or require treatment and in epidemiological monitoring to identify recovered individual’s that have previously been infected. Some chronic infections, particularly where infectious agent load rises and falls, can be hard to detect if relying purely on the detection of the infectious agent. However, a sustained antibody response may be detected8 in these individuals even when the microbe itself cannot, helping to pinpoint these individuals.
The first universal test for human immunodeficiency virus (HIV),9 developed in 1985, was an ELISA to detect human serum cystatin C. Many other diseases can be diagnosed in both humans and animals using an ELISA, including dengue fever,10 hepatitis B,11 SARS-CoV-2,12, 13 Streptococcus equi8 and Escherichia coli.14
The lipoarabinomannan (LAM) ELISA was developed to detect LAM, an immunogenic mycobacterial cell wall antigen, in the urine of tuberculosis (TB) patients. Given the global prevalence and mortality rates associated with TB and challenges of alternative TB diagnostics, it is a much-needed screening tool. However, there has been criticism of its sensitivity level15 and clinical utility.16 Recent efforts are however, working to address these problems. 17 Efforts have been made to extend the test to other sample types, including sputum18 and serum, but with less success.
Detection of food allergens
Allergens19 can be potentially fatal if ingested by affected individuals, so it is vital that any foods declared free from certain allergens, such as peanuts,20 are exactly that. The ELISA provides excellent sensitivity for this purpose, down to parts per million (ppm) in some cases, and is able to cope with some food types, such as oils and milks, that alternative methods like PCR can struggle with. However, some food processing, such as heating which can change the allergen’s conformation, can negatively impact21 detection by ELISA.
Biosimilars and biopharmaceutical testing
With the development of biosimilar drugs, there is a need to prove their similarity to the reference drugs they seek to emulate. Here too ELISAs have found utility22 in determining factors such as total drug concentration and detecting impurities.
Cancer biomarker testing
Cancer biomarkers23 are often detectable in blood samples. Therefore, ELISA testing can be utilized as a non-invasive method with which to detect and quantify known biomarkers.
Forensic drug testing
ELISAs can be used by toxicologists to screen forensic samples for traces of drugs of abuse24 including amphetamines, cocaine, opiates, methadone, benzodiazepines and cannabinoids. While liquid samples, such as urine or blood are easier, it is also possible to process solid samples, such as hair, for ELISA analysis.
Pregnancy testing
Human chorionic gonadotropin (hCG), a hormone produced in pregnancy,25 can be detected by ELISA in urine samples.
Autoimmune disease
Markers indicative of autoimmune diseases such as rheumatoid arthritis26 and lupus27 may be detected and quantified by ELISA.
References
1. Waritani T, Chang J, McKinney B, Terato K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX. 2017;4:153-165. doi:10.1016/j.mex.2017.03.002
2. López-Otín C, Bond JS. Proteases: Multifunctional enzymes in life and disease. J Biol Chem. 2008;283(45):30433-30437. doi:10.1074/jbc.R800035200
3. Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry. 1971;8(9):871-874. doi:10.1016/0019-2791(71)90454-X
4. Weemen BKV, Schuurs AHWM. Immunoassay using antigen—enzyme conjugates. FEBS Letters. 1971;15(3):232-236. doi:10.1016/0014-5793(71)80319-8
5. Lindström P, Wager O. IgG autoantibody to human serum albumin studied by the ELISA-technique. Scand J Immunol. 1978;7(5):419-425. doi:10.1111/j.1365-3083.1978.tb00472.x
6. Kato K, Hamaguchi Y, Okawa S, et al. Use of rabbit antibody IgG bound onto plain and aminoalkylsilyl glass surface for the enzyme-linked sandwich immunoassay. J Biochem. 1977;82(1):261-266. doi:10.1093/oxfordjournals.jbchem.a131678
7. Yorde DE, Sasse EA, Wang TY, Hussa RO, Garancis JC. Competitive enzyme-linked immunoassay with use of soluble enzyme/antibody immune complexes for labeling. I. Measurement of human choriogonadotropin. Clin Chem. 1976;22(8):1372-1377. doi: 10.1093/clinchem/22.8.1372
8. Robinson C, Steward KF, Potts N, et al. Combining two serological assays optimises sensitivity and specificity for the identification of Streptococcus equi subsp. equi exposure. Vet. J. 2013;197(2):188-191. doi:10.1016/j.tvjl.2013.01.033
9. Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23(4):249-253. doi:10.1128/CVI.00053-16
10. Narayan R, Raja S, Kumar S, et al. A novel indirect ELISA for diagnosis of dengue fever. Indian J Med Res. 2016;144(1):128-133. doi:10.4103/0971-5916.193300
11. Kim S-H. ELISA for Quantitative determination of hepatitis B virus surface antigen. Immune Netw. 2017;17(6):451-459. doi:10.4110/in.2017.17.6.451
12. MacMullan MA, Ibrayeva A, Trettner K, et al. ELISA detection of SARS-CoV-2 antibodies in saliva. Sci Rep. 2020;10(1):20818. doi:10.1038/s41598-020-77555-4
13. Freeman B, Lester S, Mills L, et al. Validation of a SARS-CoV-2 spike protein ELISA for use in contact investigations and serosurveillance. bioRxiv. Published online April 25, 2020:2020.04.24.057323. doi:10.1101/2020.04.24.057323
14. Tong C, Wu Z, Yu L, et al. Development of an indirect ELISA for detection of E. coli antibodies in cow serum using a recombinant OmpT as antigen. J Immunoassay Immunochem. 2014;35(3):241-255. doi:10.1080/15321819.2013.848812
15. Reither K, Saathoff E, Jung J, et al. Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC Infect Dis. 2009;9(1):141. doi:10.1186/1471-2334-9-141
16. Dheda K, Davids V, Lenders L, et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One. 2010;5(3):e9848. doi:10.1371/journal.pone.0009848
17. Amin AG, De P, Graham B, Calderon RI, Franke MF, Chatterjee D. Urine lipoarabinomannan in HIV uninfected, smear negative, symptomatic TB patients: effective sample pretreatment for a sensitive immunoassay and mass spectrometry. Sci Rep. 2021;11(1):2922. doi:10.1038/s41598-021-82445-4
18. Peter JG, Cashmore TJ, Meldau R, Theron G, van Zyl-Smit R, Dheda K. Diagnostic accuracy of induced sputum LAM ELISA for tuberculosis diagnosis in sputum-scarce patients. Int J Tuberc Lung Dis. 2012;16(8):1108-1112. doi:10.5588/ijtld.11.0614
19. Iqbal A, Farooq S, Jamal Y, et al. Detection of food allergens by ELISA and other common methods. Fresenius Environ. Bull. 2018;27(12): 8340-8346.
20. Pandey AK, Varshney RK, Sudini HK, Pandey MK. An improved enzyme-linked immunosorbent assay (ELISA) based protocol using seeds for detection of five major peanut allergens Ara h 1, Ara h 2, Ara h 3, Ara h 6, and Ara h 8. Front Nutr. 2019;6:68. doi:10.3389/fnut.2019.00068
21. Taylor SL, Nordlee JA, Niemann LM, Lambrecht DM. Allergen immunoassays--considerations for use of naturally incurred standards. Anal Bioanal Chem. 2009;395(1):83-92. doi:10.1007/s00216-009-2944-0
22. Li M, An W, Wang L, et al. Production of monoclonal antibodies for measuring Avastin and its biosimilar by Sandwich ELISA. J Immunol Methods. 2019;469:42-46. doi:10.1016/j.jim.2019.03.013
23. Dou Y, Lv Y, Zhou X, et al. Antibody-sandwich ELISA analysis of a novel blood biomarker of CST4 in gastrointestinal cancers. Onco Targets Ther. 2018;11:1743-1756. doi:10.2147/OTT.S149204
24. Agius R, Nadulski T. Utility of ELISA screening for the monitoring of abstinence from illegal and legal drugs in hair and urine. Drug Test Anal. 2014;6(S1):101-109. doi:10.1002/dta.1644
25. Podrouzek P, Krabec Z, Mancal P, Presl J. The development and evaluation of Sevatest ELISA hCG Micro I. kit as a test for pregnancy. J Hyg Epidemiol Microbiol Immunol. 1988;32(4):467-476. PMID: 3221094
26. Suzuki K, Sawada T, Murakami A, et al. High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol. 2003;32(4):197-204. doi:10.1080/03009740310003677
27. Dillon SP, D’Souza A, Kurien BT, Scofield RH. Systemic lupus erythematosus and C1q: A quantitative ELISA for determining C1q levels in serum. Biotechnol J. 2009;4(8):1210-1214. doi:10.1002/biot.200800273