Antimicrobial Resistance
Complete the form below to unlock access to ALL audio articles.
Antimicrobial resistance (AMR) is the ability of microorganisms (including bacteria, fungi, viruses and parasites) to resist the killing capacity of antimicrobial drugs (such as antibiotics, antifungals, antivirals, antimalarials and anthelmintics).
Whilst AMR incorporates resistance of all classes of microbes to antimicrobial treatments, the major focus in research has been on antibiotic resistance in bacteria. This is because bacterial infections are responsible for the bulk of community-acquired and nosocomial infections. This is compounded by the fact that the molecular tools available for the study of resistance mechanisms are more well-established in bacteria than in other microbes, and the sheer number of antibacterial classes offers greater scope for studying modes of action and resistance.
Therefore, in the rest of this article, we are going to focus on antibiotic resistance in bacteria.
Section quick links
The science behind antimicrobial resistance
Drivers of antimicrobial resistance development and spread
Testing for antimicrobial resistance
AMR prevention
Alternatives to antimicrobials
The science behind antimicrobial resistance
Antibiotics are divided into two broad classes, bacteriostatic and bactericidal, and work through a number of actions, including:
- Inhibition of cell wall synthesis (beta-lactams, vancomycin)
- Inhibition of protein synthesis (aminoglycosides, tetracyclines, chloramphenicol, macrolides)
- Alteration of cell membranes (polymyxins)
- Inhibition of nucleic acid synthesis (quinolones, metronidazole, rifampicin)
- Interference with metabolism (sulfonamides, trimethoprim)
In order to be resistant, a bacterium must be able to work around or prevent the action of antibiotics. This they achieved by a number of routes, including:
Preventing the antibiotic reaching its target at a sufficiently high concentration
- Efflux pumps pump antibiotics out of the cell
- Reduced membrane permeability prevents antibiotic entry
- Enzymes destroy the antibiotic, e.g. β-lactamase against penicillin
- Enzymes modify the antibiotic, adding chemical groups that inhibit its activity
Modifying or bypassing the target on which the antibiotic acts
-Produce alternative proteins that can be used instead of the ones targeted by the antibiotic
- Alter the target of the antibiotic so it can no longer interact with it
To achieve the resistance mechanisms described, the bacterium requires the correct tools (proteins) for the job, and that requires the correct genes.
AMR may arise naturally as a result of genetic mutations or “mistakes” that occur during replication. Bacteria are also able to acquire genetic material from other bacteria through a process known as horizontal gene transfer.
Horizontal transfer of genes can occur via three mechanisms, transformation, transduction and conjugation. Transformation occurs when bacteria take up free DNA from their surrounding environment. This DNA may be in the form of plasmids. In transduction, bacteria are attacked by viruses called bacteriophage that can integrate themselves into the bacterial genome. When they excise themselves from the host genome to reproduce, they can take sections of host DNA with them too. When they then integrate into a new host genome, they therefore ferry bacterial genes between the bacteria, some of which may confer antibiotic resistance. Conjugation is bacteria’s version of “sex”. Sex pili are formed between bacterial cells, through which they exchange genetic material.
Under normal conditions a mutation or the addition of genetic material that confers resistance to antibiotics may offer no benefit, and in some cases, organisms that have gained resistance genes can be less fit due to the extra energetic burden of reproducing additional genetic material. Consequently, they may be lost from the population and even selected against. However, in the presence of antibiotics, the picture changes. Those who are resistant have a selective advantage and are able to persist and replicate, passing on resistance to the next generation, while susceptible competitors are killed off. This positive selection drives the spread of antibiotic resistance through a population in the face of antibiotic exposure.
Altering bacterial mode of growth can also confer resistance to antibiotic treatment. Slowed growth rates, sometimes called persistor cells, mean that antibiotics that rely on interrupting cell replication are less effective. Bacterial biofilms also offer protection from antibiotic action. Biofilms are a structured community of bacteria amid a self-made hydrated polymeric matrix with channels for nutrient acquisition and waste disposal. The structure provides physical protection as antibiotics can struggle to reach the heart of a developed bacterial biofilm. Cell replication is often slowed in a biofilm mode of growth which offers protection from cell cycle-dependent action too.
Serious problems come when microbes become resistant to multiple drugs, known as multidrug resistance. This may be because a single resistance mechanism confers resistance to multiple drugs, or if multiple resistance genes are acquired, singularly or in combination.
AMR is present in every country in the world. Approximately 700,000 deaths per year globally are attributed to AMR, but this figure is predicted to rise to 10 million by 2050, posing a significant global concern. Reducing the occurrence of AMR and preventing its spread is therefore vital in protecting both people and animals.
Drivers of antimicrobial resistance development and spread
Whilst AMR may occur naturally, there are a number of factors that can help to promote AMR occurrence and spread.
Inappropriate handling of contaminated water and waste at pharmaceutical manufacturing plants can see water discharged into the environment that is contaminated by antibiotics. Whilst the water itself may not harm you, it exposes bacteria in the environment to antibiotics, promoting the development and spread of resistance through the bacterial population. These resistant strains may then infect animals if they come into contact with them, e.g. through consumption, contamination of other foodstuffs or swimming.
Sewerage is another key source of motivation for bacteria to develop antibiotic resistance. When humans or animals are given antibiotics, not all of them will be processed by the body, and the remainder will be excreted in feces. These “left-over” antibiotics expose the mixing pot of microbes in our sewer system, or in the case of animals their environment and cohabitants, to antibiotics, applying pressure to gain resistance. Whilst sewerage treatment removes many unwanted contaminants, currently this does not include antibiotics. Consequently, “treated sewerage”, discharged, recycled, used for irrigation or for fertilizer, may contain antibiotics. Finding an effective and sustainable removal method has become an active area of research.
Many food-producing animals are prophylactically treated with antibiotics to try and keep them healthy. However, this means that the naturally occurring bacteria in their gut, which can include species such as Staphylococcus aureus, are exposed to antibiotics. This promotes the survival and spread of resistant strains which may then contaminate the environment they live in, personnel that handle them and the food produced from them. If contaminated feces enter the water or are used as fertilizer, crops too can become contaminated.
Factors such as poor hygiene and overcrowding are just the conditions antimicrobial resistance needs to spread between humans, animals and the environment, so employing effective preventative measures are key.
Testing for antimicrobial resistance
There are a variety of testing strategies that can be performed, both phenotypic and molecular, that enable scientists to determine which antibiotics a given strain of bacteria is sensitive to and which it is not.
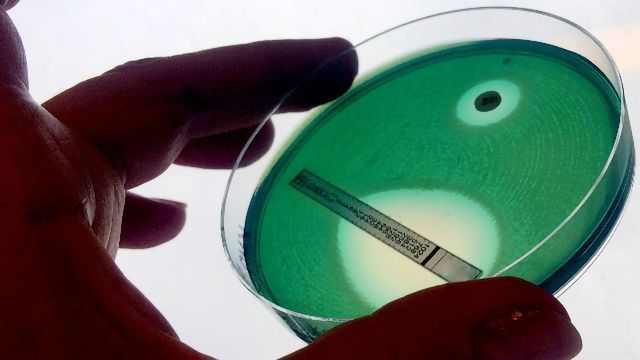
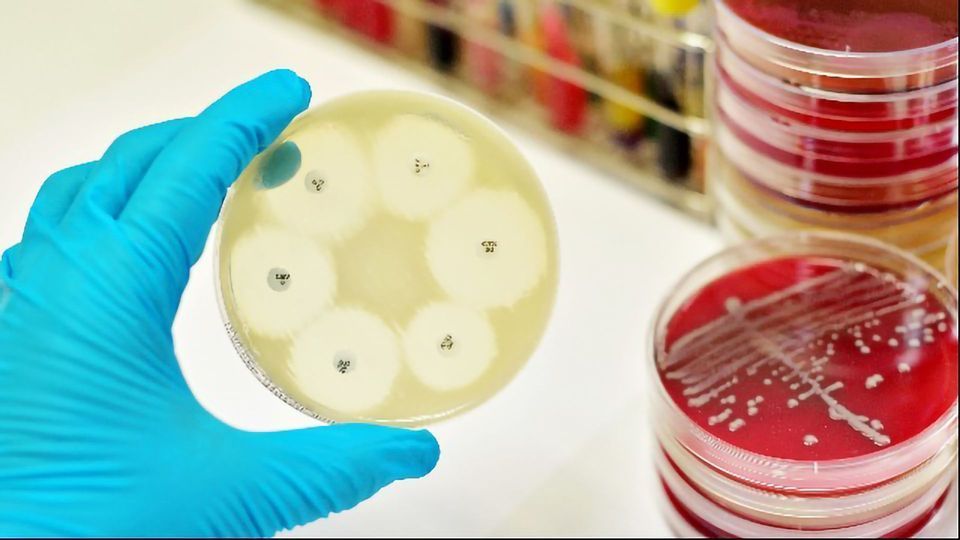
Sensitivity testing, in which a lawn of the bacterial strain of interest is cultured on agar plates onto which antibiotic-infused discs are placed has been a popular phenotypic screening technique for many years and remains the gold standard. The larger the area of clearance around a disc, the more sensitive the strain is to that particular antibiotic. Microfluidic devices designed to look at the minimum inhibitory concentration of antibiotics needed to kill a strain have been used to the same end. However, the downside of methods requiring culture is the length of time it can take to isolate, culture and then test the sensitivity of a strain, often taking 48 hours of more to draw a conclusion. When treating aggressive infections this can be too long.
Major advances in the last decade have seen whole genome sequencing (WGS) take its place in the study and fight against AMR. WGS enables known and unknown AMR-related genes to be identified and their propagation through a population monitored. Multiple mutations or genes may confer the same resistance which is not typically differentiated by phenotypic methods. WGS provides a much more detailed picture of resistance progression and spread and has enabled the chains of resistant infection spread to be mapped precisely, which is helpful in monitoring studies.
Amplification tests, such as PCR, RT-qPCR and LAMP assays, can offer a quick and specific solution to the detection of resistance genes. However, they are only able to identify known targets and further mutations within the area target by the assay can render it ineffective.
Hybridization assays, such as arrays, fluorescent in situ hybridization (FISH) and line probe assays (LPAs), rely on the hybridization of probes to target DNA sequences which can then be detected using techniques such as fluorescence. As with amplification-based tests, they require prior knowledge of target mutations and will not identify any novel AMR-conveying targets.
Immunoassays, including lateral flow tests and enzyme-linked immunosorbent assays (ELISAs), can be useful for AMR detection too. The binding of antibodies either to the target gene or gene product can be then be used to detect their presence, usually via conjugation to a visible indicator.
There is a real push towards rapid point-of-care testing to which assays like lateral flow and some microfluidic devices lend themselves, enabling clinicians to overcome some of the current antimicrobial stewardship challenges and prescribe appropriately.
Funding opportunities are vital in facilitating research to improve the diagnostics available, promoting the development of affordable, accurate, fast and easy-to-use tests for bacterial infections.
No matter what techniques are used for bacterial isolation and identification, and which methods of resistance testing are employed, it is important that bacteria are cultured appropriately and handled safely. Good aseptic technique is essential to protect yourself and your sample.
AMR prevention
It is vitally important that steps are taken to prevent further development and spread of AMR. Simple hygiene precautions, such as handwashing, appropriate handling of food items and using clean water can help to reduce the likelihood of contracting a resistant infection. However, they do not avoid the pressure exerted by antibiotic exposure on microbes that drive the development of AMR in the first place.
Limiting the exposure of bacteria to antibiotics is important and there are a number of measures that can help.
Antibiotic stewardship - the effort to measure and improve how antibiotics are prescribed by clinicians and used by patients - is at the heart of reducing AMR. This includes the use of narrow rather than broad spectrum antibiotics to target the infection more specifically, use only when necessary and only for as long as necessary. Responsibility does not all fall to medics and vets, the public also have their part to play to ensure that they (or their animals) only take the antibiotics prescribed to them, at the prescribed dose, at the advised times and that they complete the course.
Reducing prophylactic use of antibiotics, especially in the food animal industry, is key in preventing unnecessary exposure of both the body’s own bacteria and those that come into contact with antibiotic-containing feces.
Measurement of procalcitonin, the precursor to the hormone calcitonin, via immunoassay has been shown to be a good indicator of bacterial infection where levels become elevated. These indicators aid treatment decision making for clinicians, and consequently antibiotic stewardship. Where infections are suspected or progression to sepsis is a risk, antibiotic stewardship promotes appropriate use of, and aims to reduce the number of days on, antibiotic therapy. Rapid multiplex PCR has also been put to use in distinguishing bacterial from viral infections to guide antibiotic prescribing.
Whilst we may still not have an answer to antibiotics entering the environment from domestic effluent, stricter monitoring and enforcement of the wastewater discharged by pharmaceutical companies can help to prevent antibiotics from entering the environment and water system.
AMR monitoring programs are vital in keeping diagnostics up-to-date as new AMR targets emerge and identifying patterns from which remedial plans can be actioned. Artificial intelligence is also playing its part, helping to monitor microbes in real time and predict AMR and antibiotic susceptibility.
Scientists are working on ways to remove antibiotics from contaminated soils using engineered bacterial strains capable of breaking down antibiotics as a bioremedial agent. Removal from wastewater is still proving challenging but researchers are tackling the issue.
Alternatives to antimicrobials
With the frequency with which bacterial strains are exhibiting resistance growing, even to antibiotics of last resort, scientists are looking for new drugs and alternatives to antimicrobial treatment.
The discovery of “new antibiotics” however is not trivial, compounded by financial and regulatory disincentives, leading scientists to look elsewhere for solutions.
Phage therapy was explored as a method for treating bacterial infections prior to the discovery of penicillin in 1928, after which it was all but abandoned. Soviet nations continued research in the area, due to a lack of access to western antibiotics. Now, as we struggle to combat infection with antibiotics, interest has once more peaked in phage therapy. Whilst phage are able to infect and destroy bacteria, they are species and even strain specific. Added to which the potential for pre-existing immunity of some bacteria mean targeting a phage treatment is currently not a trivial process and must be tailored to the individual infection, making it costly and lengthy.
CRISPR-Cas9 has garnered much attention in the field of gene therapy, however it may also have a role in antimicrobial therapy. Currently more work is required.
Antimicrobial peptides have thus far shown limited success, however synthetic peptides and synthetic membrane-active agents may offer future avenues for exploration.
Antibody therapy is an expensive but highly specific alternative, but one which has been implemented in part.
Nitric oxide is a natural antimicrobial and plays a critical role in our immune response to pathogens. Consequently, scientists are exploring how it may be incorporated into treatment regimes.
The use of antibiotic resistance breakers (ARB) are also being explored, which involves co-administering a non-antibiotic drug with a failing antibiotic.
In the meantime, we all have our part to play in the prevention of AMR development and spread.