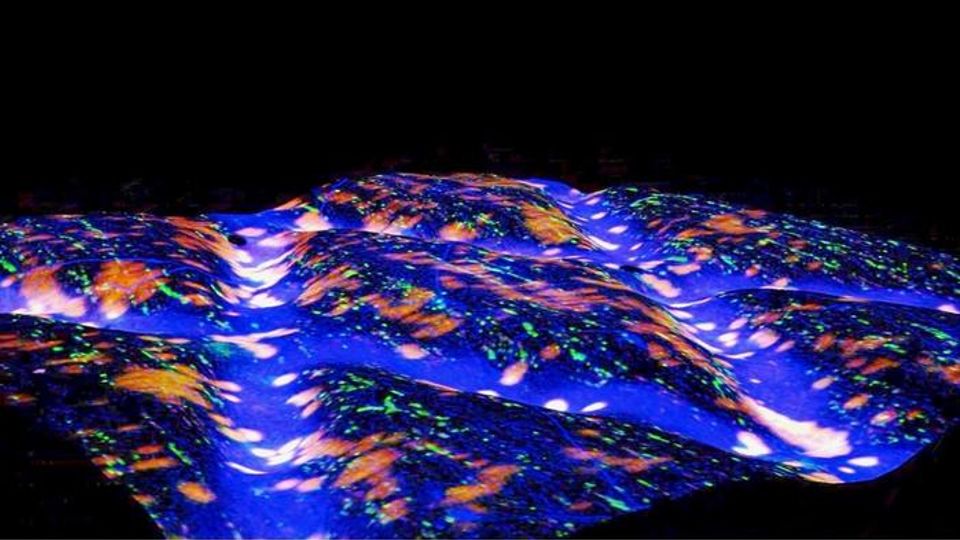
Biologic “Patch” Activated by Natural Motion Could Help Fix Herniated Discs
The tension-activated repair patch plugs holes in discs in the spine like car tire patches.
Complete the form below to unlock access to ALL audio articles.
A new biologic “patch” that is activated by a person’s natural motion could be the key to fixing herniated discs in people’s backs, according to researchers at the Perelman School of Medicine at the University of Pennsylvania and the CMC VA Medical Center (CMCVAMC). Combining years of work from many different projects, the “tension-activated repair patches” (TARPs) provide controlled release of an anti-inflammatory molecule called anakinra from microcapsules over time, which helped discs in a large animal model regain the tension they need to reverse herniation and prevent further degeneration. This pre-clinical research is detailed in a paper published today in Science Translational Medicine.
“Currently there is no curative treatment for disc herniation, and the best thing out there is just like sticking a plain rubber plug into a hole in a tire. It will stay for a while but it won’t make a great seal,” said co-senior author Robert Mauck, PhD, a professor in Orthopaedic Surgery and director of the McKay Laboratory for Orthopaedic Surgery Research at Penn and research career scientist and co-director of the Translational Musculoskeletal Research Center at the CMCVAMC. “The patch we’ve developed is like the plug plus glue, so you’re actually bonding the patch. And since biomechanical movement activates the patch and makes it seal more strongly, it’s like having your tire patch get stronger the more miles you put on it.”
Herniation in the spine occurs when one of the soft discs that sits between the vertebrae develops a split or a hole, and the soft interior squeezes through. This means that the discs lose their tension and are unable to cushion the spine as usual, causing pain. To continue the tire analogy, it’s as if a tire has gone flat and the car is riding on its rim.
Want more breaking news?
Subscribe to Technology Networks’ daily newsletter, delivering breaking science news straight to your inbox every day.
Subscribe for FREESo the Penn Medicine and CMCVAMC researchers have developed TARPs to not just plug the hole, but allow tension to build back up, and re-cushion the vertebrae. That goal has been particularly tough to achieve to this point.
“The disc is a very complex tissue, which is different from muscle and skin in that it cannot heal its own structure and, in fact, continues to degenerate over time once its structure is compromised,” said Ana Peredo, PhD, who completed this research during her doctoral studies in Bioengineering at the School of Engineering and Applied Sciences at Penn. “We set out to recover the disc’s mechanical integrity while simultaneously attenuating inflammation in order to prevent further tissue damage and retain as much tissue function as possible.”
Key to the TARP is having the body’s natural mechanics work to activate the release of anti-inflammatory molecules from the microcapsules within the patch. While they would theoretically still work if a person lay totally still for months, the reality of the disc tissue environment is that movement is its natural state.
And because the patch makes it as if there was never a hole to begin with, its application could have significant effects on the prevention of worsening pain related to disc degeneration.
“This is designed to be an early intervention that may change the course of disease progression,” said co-senior author Harvey Smith, MD, an associate professor of Orthopaedic Surgery and attending physician at the CMCVAMC. “Currently there’s no treatment to mitigate recurring herniations that actually heal the disc. So we’re looking at a disease that is very common in younger, working-age people that, downstream, leads to severe disc disease and the need for spinal fusion. The more we can prevent that, the better.”
This new prospective treatment, which has human and potentially veterinary applications, builds upon many years of research in the McKay Lab and at Penn, at the Translational Musculoskeletal Research Laboratory at the CMCVAMC, and within the Institute for Medical Translation at the New Bolton Center, and leverages foundational technologies used by many of the same researchers on this project to create bio-synthetic discs and other mechanically-activated drug delivery systems. Some of these advances are now being commercialized by Mechano Therapeutics, LLC , which was co-founded by Mauck and other co-authors on the current paper, George Dodge, PhD, previously an adjunct associate professor of Orthopaedic Surgery and the current CEO of the start-up, and Daeyeon Lee, PhD, a Penn Engineering professor, and supported by the Penn Center for Innovation.
“I have been working with emerging non-fusion spine technologies for 20 years, with very few making it to human clinical trials and beyond,” said Thomas Schaer, VMD, a veterinary surgeon and Director of the Institute for Medical Translation, New Bolton Center, at the Penn School of Veterinary Medicine. “This team has been working together for 15 years, and I believe we are practicing a highly focused approach to research that has significant potential for a translational breakthrough across a broad spectrum of spine care, not just for human patients but possibly also for our furry dog friends.”
While this research was primarily “proof of principle,” moving this treatment closer to the clinic will require longer trials in large animal models, the team said.
“This study was incredibly promising but went for one month, so we want to test for a longer time because there are ways we can fine-tune this patch,” said co-lead author Sarah Gullbrand PhD, a research assistant professor of Orthopaedic Surgery at Penn and research health scientist at the CMCVAMC. “We only targeted one biologic pathway this time using something that was already approved by the FDA, but there are tons of other factors that are approved. In the future, we’re interested in not only reducing inflammation, but also preventing cell death and improving overall healing.”
Reference: Peredo AP, Gullbrand SE, Friday CS, et al. Tension-activated nanofiber patches delivering an anti-inflammatory drug improve repair in a goat intervertebral disc herniation model. Sci Trans Med. 15(722):eadf1690. doi: 10.1126/scitranslmed.adf1690
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source.